what does it mean for a molecule to be ionic
Ionic and Covalent Bonds
- Folio ID
- 839
There are many types of chemic bonds and forces that bind molecules together. The two most basic types of bonds are characterized as either ionic or covalent. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at to the lowest degree one electron donor and one electron acceptor. In contrast, atoms with the same electronegativity share electrons in covalent bonds, because neither atom preferentially attracts or repels the shared electrons.
Introduction
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a blazon of chemic bail that generates two oppositely charged ions. In ionic bonds, the metal loses electrons to become a positively charged cation, whereas the nonmetal accepts those electrons to become a negatively charged anion. Ionic bonds crave an electron donor, often a metal, and an electron acceptor, a nonmetal.
Ionic bonding is observed because metals have few electrons in their outer-well-nigh orbitals. By losing those electrons, these metals can accomplish noble gas configuration and satisfy the octet rule. Similarly, nonmetals that accept close to 8 electrons in their valence shells tend to readily have electrons to achieve element of group 0 configuration. In ionic bonding, more than than 1 electron can be donated or received to satisfy the octet dominion. The charges on the anion and cation stand for to the number of electrons donated or received. In ionic bonds, the internet charge of the compound must be zilch.
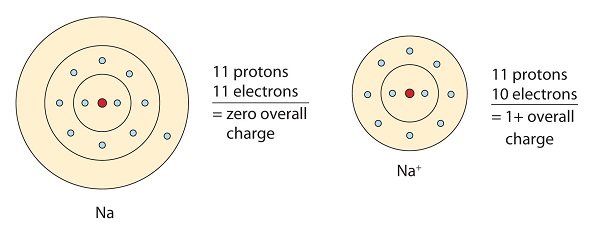
This sodium molecule donates the solitary electron in its valence orbital in order to achieve octet configuration. This creates a positively charged cation due to the loss of electron.
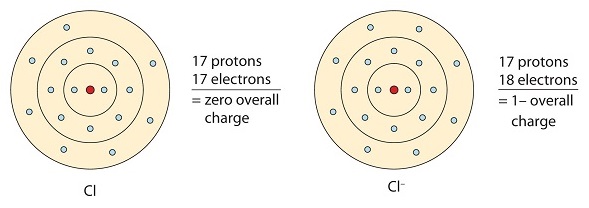
This chlorine atom receives one electron to reach its octet configuration, which creates a negatively charged anion.
The predicted overall free energy of the ionic bonding process, which includes the ionization energy of the metallic and electron analogousness of the nonmetal, is usually positive, indicating that the reaction is endothermic and unfavorable. However, this reaction is highly favorable because of the electrostatic allure between the particles. At the ideal interatomic distance, attraction between these particles releases enough free energy to facilitate the reaction. Most ionic compounds tend to dissociate in polar solvents because they are often polar. This phenomenon is due to the opposite charges on each ion.
Example \(\PageIndex{1}\): Chloride Salts
.jpg?revision=2)
In this example, the sodium atom is donating its 1 valence electron to the chlorine atom. This creates a sodium cation and a chlorine anion. Observe that the internet charge of the resulting compound is 0.
.jpg?revision=2)
In this example, the magnesium cantlet is donating both of its valence electrons to chlorine atoms. Each chlorine atom can just accept 1 electron before it can achieve its noble gas configuration; therefore, two atoms of chlorine are required to accept the 2 electrons donated by the magnesium. Notice that the internet charge of the compound is 0.
Covalent Bonding
Covalent bonding is the sharing of electrons between atoms. This type of bonding occurs between two atoms of the same element or of elements close to each other in the periodic table. This bonding occurs primarily between nonmetals; however, it can likewise be observed between nonmetals and metals.
If atoms have similar electronegativities (the same affinity for electrons), covalent bonds are most probable to occur. Considering both atoms accept the same affinity for electrons and neither has a tendency to donate them, they share electrons in guild to achieve octet configuration and become more stable. In addition, the ionization energy of the atom is too large and the electron affinity of the cantlet is too small for ionic bonding to occur. For example: carbon does non form ionic bonds because information technology has 4 valence electrons, half of an octet. To form ionic bonds, Carbon molecules must either gain or lose 4 electrons. This is highly unfavorable; therefore, carbon molecules share their 4 valence electrons through single, double, and triple bonds and so that each cantlet can attain element of group 0 configurations. Covalent bonds include interactions of the sigma and pi orbitals; therefore, covalent bonds lead to formation of unmarried, double, triple, and quadruple bonds.
Instance \(\PageIndex{ii}\): \(PCl_3\)
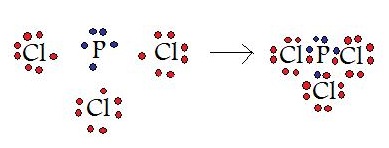
In this example, a phosphorous atom is sharing its three unpaired electrons with three chlorine atoms. In the cease product, all four of these molecules have 8 valence electrons and satisfy the octet dominion.
Bonding in Organic Chemical science
Ionic and covalent bonds are the 2 extremes of bonding. Polar covalent is the intermediate type of bonding betwixt the 2 extremes. Some ionic bonds contain covalent characteristics and some covalent bonds are partially ionic. For example, nigh carbon-based compounds are covalently bonded simply tin also be partially ionic. Polarity is a mensurate of the separation of charge in a chemical compound. A compound'due south polarity is dependent on the symmetry of the compound and on differences in electronegativity betwixt atoms. Polarity occurs when the electron pushing elements, found on the left side of the periodic table, exchanges electrons with the electron pulling elements, on the right side of the tabular array. This creates a spectrum of polarity, with ionic (polar) at one extreme, covalent (nonpolar) at another, and polar covalent in the center.
Both of these bonds are important in organic chemistry. Ionic bonds are important because they allow the synthesis of specific organic compounds. Scientists can dispense ionic properties and these interactions in order to form desired products. Covalent bonds are peculiarly of import since almost carbon molecules interact primarily through covalent bonding. Covalent bonding allows molecules to share electrons with other molecules, creating long chains of compounds and allowing more than complexity in life.
References
- Vollhardt, K. Peter C., and Neil East. Schore. Organic Chemistry Construction and Function. New York: West. H. Freeman, 2007.
- Petrucci, Ralph H. General Chemistry: Principles and Modernistic Applications. Upper Saddle River, NJ: Pearson Education, 2007.
- Chocolate-brown, Theodore L., Eugene H. Lemay, and Bruce East. Bursten. Chemistry: The Central Science. sixth ed. Englewood Cliffs, NJ: Prentice Hall, 1994.
Problems
1. Are these compounds ionic or covalent?

2. In the following reactions, betoken whether the reactants and products are ionic or covalently bonded.
a)
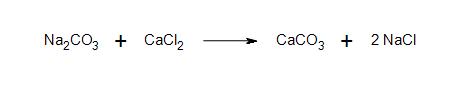
b) Description: What is the nature of the bond between sodium and amide? What kind of bond forms between the anion carbon chain and sodium?
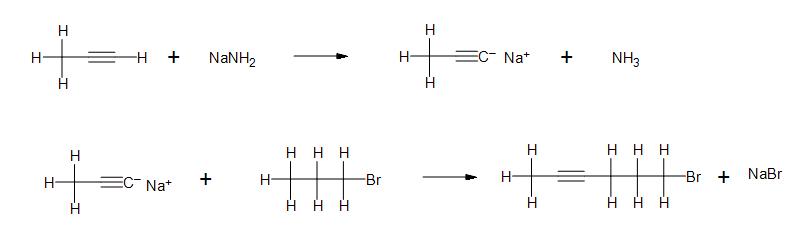
c)
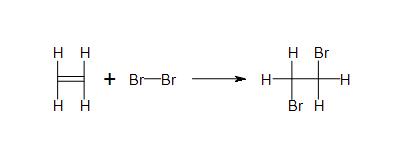
Solutions
- ane) From left to right: Covalent, Ionic, Ionic, Covalent, Covalent, Covalent, Ionic.
- 2a) All products and reactants are ionic.
- 2b) From left to right: Covalent, Ionic, Ionic, Covalent, Ionic, Covalent, Covalent, Ionic.
- 2c) All products and reactants are covalent.
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_%28Organic_Chemistry%29/Fundamentals/Ionic_and_Covalent_Bonds
0 Response to "what does it mean for a molecule to be ionic"
Post a Comment